The Medical Devices Unit of SAHPRA regulates the licencing of medical device establishments and the registration of medical devices (In vitro diagnostics (IVDs) and non-IVD medical devices) in South Africa to ensure the availability of medical devices that comply with an acceptable level of safety, quality and performance.
ADMINISTRATION
Licensing of Medical Device Establishments
- An applicant may apply for one of three types of licences for medical device establishments: manufacturer (manufacture, pack, label, service, import, export), distributor (import, export, distribute) and wholesaler (storage, transportation, delivery).
- A call up notice, published in Government Gazette No. 40637, on the 24 February 2017, requested all manufacturers and distributors of medical devices to apply for a SAHPRA licence within 6 months of publication of said notice and wholesalers were required to apply within 12 months of said notice.
Currently, a transitional arrangement allows for the use of an acknowledgement letter received from SAHPRA, in lieu of a medical device establishment licence, on submission of a licence application, so as to facilitate trade and ensure continuation of business and access to medical devices whilst the SAHPRA licensing process developed. As of the 31 March 2020, the use of an acknowledgement letter in lieu of a medical device establishment licence will not be permitted.
No medical device may be manufactured, distributed, imported, exported or sold without a valid SAHPRA medical device establishment licence.
- Note 1: Providing evidence of a valid SAHPRA medical device establishment licence will be required to be eligible to bid for National and Provincial tenders.
- Note 2: A manufacturer, distributor, wholesaler of a non-sterile, non-measuring Class A medical device is exempt from licencing, as per the SAHPRA position statement 9.106 “Class A medical devices”.
Medical device establishments who have applied for a SAHPRA licence must appoint an Authorised Representative who must be a natural person based in South Africa. The representative is responsible for adherence to the law, regulations and guidelines.
As part of the application for a SAHPRA medical device establishment licence, a company must list all the medical devices that it manufactures, distributes, or wholesales. The application includes a declaration regarding the status of the quality management system in place in the company. Upon renewal of the SAHPRA licence, manufacturers and distributors will have to provide evidence of ISO 13485 certification for the company by an accredited conformity assessment body.
Registration of Medical Devices
The registration process for medical devices is still in development.
A Registration Call-Up Plan will be published to inform stakeholders of the phased approach in which medical devices will be called up for registration. This plan will specify the type and class of medical devices that will be prioritised and called up sequentially. SAHPRA will implement reliance pathways in the registration of medical devices based on the verification of registration of medical devices in other recognised jurisdictions including Australia, United States, European Union, Brazil, Canada, Japan and or pre-qualification of IVDs by the World Health Organisation.
FEES
FAQs
PROCESSES
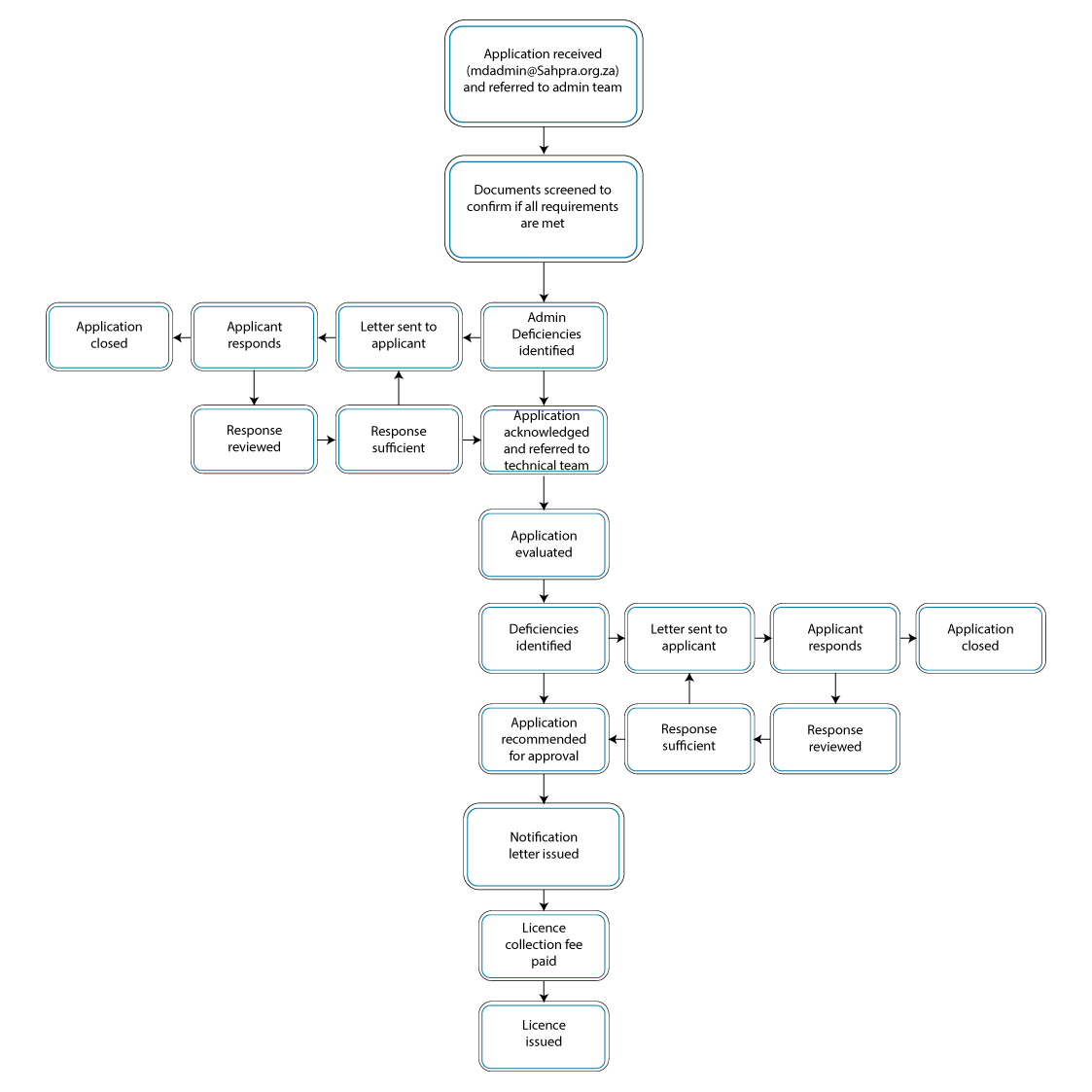
PROCESS FLOW
LEGISLATION