
1. Purpose
The Clinical Trial Unit of the South African Health Products Regulatory Authority (SAHPRA) provides legal framework for the review of clinical trials and Bioequivalence studies for human participants and recommends approval of the conduct of clinical trials. The unit receives, processes and evaluates the applications from applicants (industry, academia and investigators) for approval to conduct the study within South Africa (SA). The unit also provide for authorisation for the importation of unregistered medicine for the purpose of conducting clinical trials. Any amendments required during the conduct of the study, must be approved by SAHPRA.
2. Legislative Framework
Medicines and Related Substance Act, Act 101 of 1965, provides for the legislative framework for access to unregistered medicines, it is enabled by Section 21 of the Act as amended: Authority may authorize sale of unregistered medicines for purpose of conducting clinical trials.
South African Good Clinical Practice Guidelines (SA GCP) provide researchers and other interested parties with clearly articulated standards of GCP in locally conducted research that address the local realities and contexts, to ensure that clinical trials involving South African human participants are designed and conducted according to local requirements as well as according to the sound scientific and ethical standards within the accepted framework for good clinical practice.
SAPRA has developed several guidelines for researchers in order to ensure that human participants are protected and are able to derive benefits from participating in clinical trials conducted in South Africa.
3. Processing Clinical Trials Applications
-
- Researchers must submit a completed application on predetermined dates and obtain proof of delivery. An application form must be accompanied by the prescribed fee. The proof of delivery, proof of payments and cover page must be sent to SAHPRA via email.
-
-
- SAHPRA New Clinical Trials Process
- Pre-Approval
- Post-Approval
- Processing of Application for Protocol Amendment and Additional Sites And/Investigators
-
SAHPRA New Clinical Trials Process / Pre-Approval
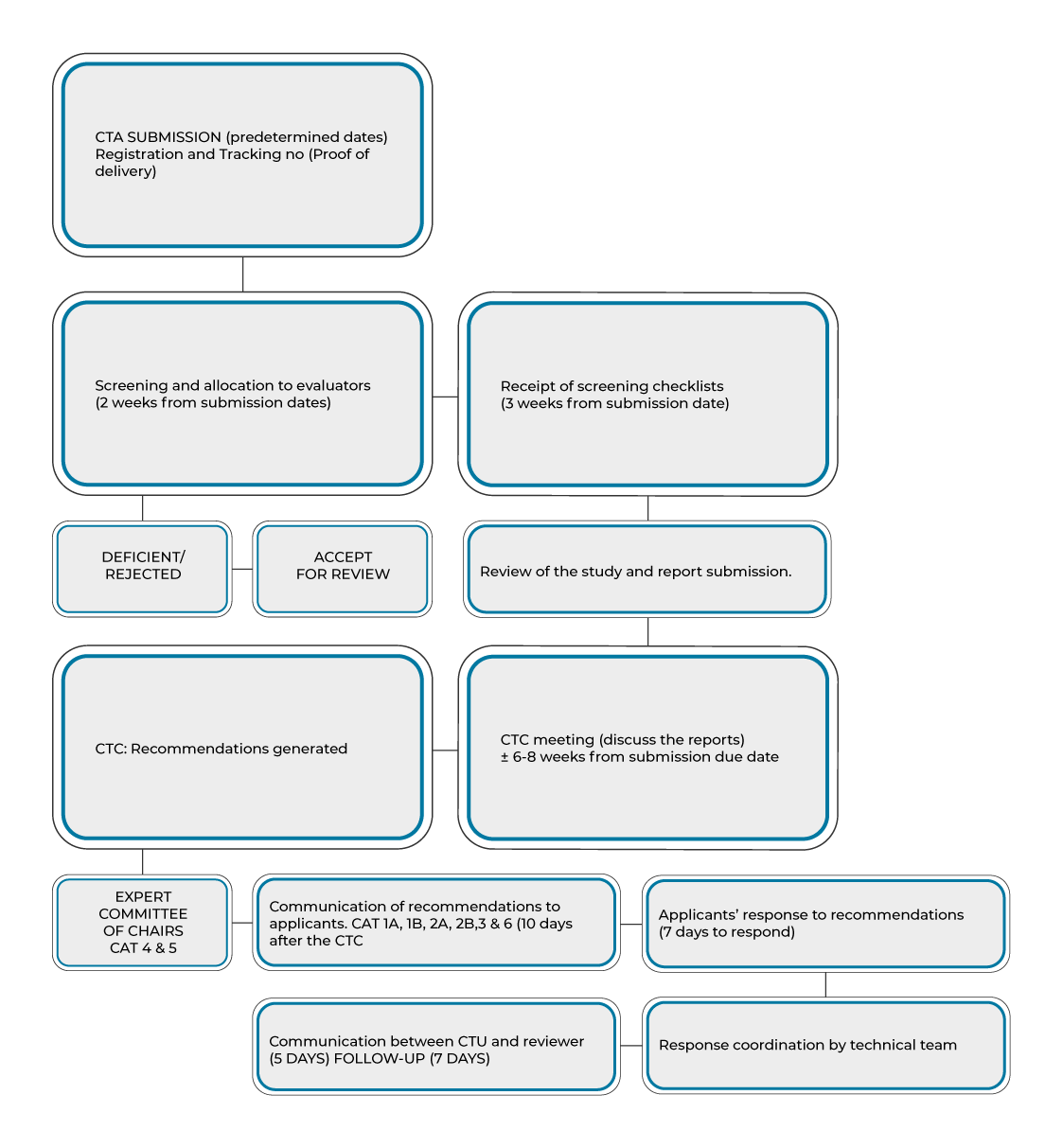
4. Turnaround Time for Response to a Clinical Trial Application
Clinical Trial Unit aim to process new applications and issue checklist within 3 weeks of receipt. The Clinical Trial Committee recommendations would be sent within 10 weeks of submission due date. There are cases where this turnaround time might be prolonged i.e. unfamiliar investigational product which may be referred to external reviewers or other committees of SAHPRA for input.
The timeline to receive the response after the submission of application for additional investigators, site(s) and protocol amendment is about 6 weeks following receipt of the application. The applicants should make use of the electronic submission process.
5. Fees Payable to SAHPRA
- Details of all clinical trials application whereby fees are applicable are available on: Fees payable to SAHPRA
6. SANCTR
-
- The South African National Clinical Trials Register (SANCTR) provides the public with updated information on clinical trials on human participants being conducted in South Africa. The Register provides you with information on a trials purpose; who can participate, where the trial is located, and contact details. Find out more.
7. Contact us
- New clinical trials application alert, Responses to new Clinical Trial applications and related queries: ctcresponses@sahpra.org.za
- Protocol amendments, responses to amendments and related queries: ctcamendments@sahpra.org.za
- Additional Investigators & sites, responses to additional and related queries: ctcinvestigators@sahpra.org.za
- Bioequivalence studies, BE amendments, responses to BE studies and related queries: ctcbeprotocols@sahpra.org.za
- Notifications and related queries: ctcnotifications@sahpra.org.za
- Individual Patient Serious Adverse Events and related queries: ctcsaes@sahpra.org.za
- SAHPRA guidelines, forms and related queries: CTCGuidelines@sahpra.org.za




